

An ergonomic breast biopsy system with Single Insertion Multiple Sample (SIMSTM) technology
Reliable performance
Diagnostic quality sample: Consistently capture samples you need for diagnosis.
Smart mode: Engages if additional sample sequences are required for tissue transport.
Optional firing—20mm: Provides additional placement control.
Echogenicity: Echogenic markings to visualize sample notch under ultrasound.
Efficiente procedures
SIMS: Reliably capture multiple samples with a single insertion.
Sample time: Approximately 9 seconds per sample*.
Reduced waste: Requires no additional accessories such as tubing and cannisters.
Ease of set-up and breakdown: Quick and intuitive set-up process and easy clean-up.
Versatile usability
Ergonomic: Operates intuitively, with easy-to-identify buttons and ergonomic design.
Needle-sharpness: The probe features the sharp TriConcave™ tip, designed for ease of penetration.
Gauge sizes: Choice of 10G, 12G, and 14G sizes for the flexibility to handle.
Integrated coaxial: Compatible with 10cm BD breast markers.
*NOTE: Average sample time was observed in a preclinical model. Preclincial testing may not be predictive of actual clinical outcomes. Different test methods may yield different results. Data on File. Bard Peripheral Vascular, Inc., Tempe, AZ.
BD EleVation™ Breast Biopsy System
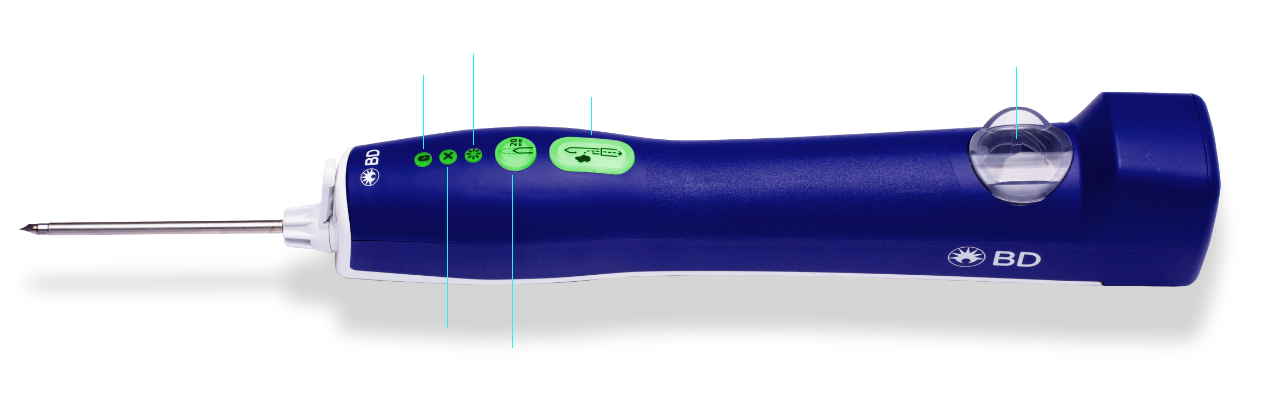
Battery
Indicates power level
Smart mode
Alerts physician to dense or difficult tissue
Error indicator lights
Alerts users of error
Optional firing
20mm
Sample
Activates sampling sequence with single press

Sample container
• Illuminated sample container
• Enables you to visualize sample tissue for faster clinical decision-making
• Easy to remove and fits into a standard formalin container for transfer to pathology lab
BD EleVation™
Breast Biopsy System
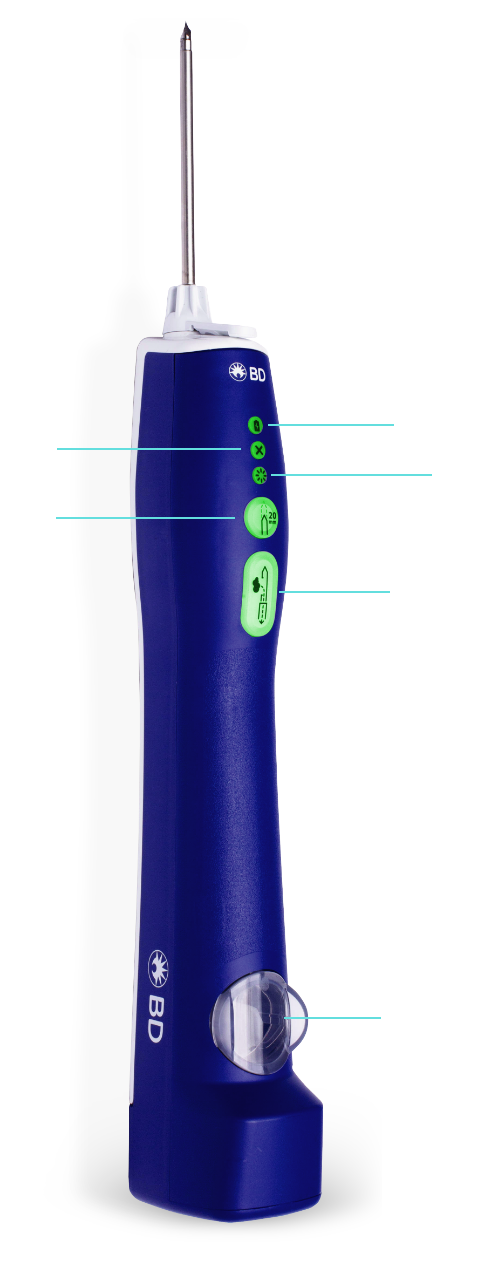
Battery
Indicates power level
Smart mode
Alerts physician to dense or difficult tissue
Error indicator lights
Alerts users of error
Optional firing
20mm
Sample
Activates sampling sequence with single press

Sample container
• Illuminated sample container
• Enables you to visualize sample tissue for faster clinical decision-making
• Easy to remove and fits into a standard formalin container for transfer to pathology lab
Provides better control of device during a procedure.
Lightweight, low-profile driver
Well-balanced and ergonomic to facilitate sampling in all clock positions and easy access to lesions in all locations of the breast. Durable to withstand repeated torque, reasonable drops, and multiple cleanings without damage or discoloration.
Choice of cannula for procedural flexibility
Choice of gauge sizes (10G, 12G and 14G).
Half-notch support cannula reduces sample notch from 2cm to 1cm.
Removable support cannula, compatible with 10cm BD breast markers facilitates marker placement.
Three probe sizes available



Provides better control of device during a procedure.
Lightweight, low-profile driver
Well-balanced and ergonomic to facilitate sampling in all clock positions and easy access to lesions in all locations of the breast. Durable to withstand repeated torque, reasonable drops, and multiple cleanings without damage or discoloration.

Choice of cannula for procedural flexibility
Choice of gauge sizes (10G, 12G and 14G).
Half-notch support cannula reduces sample notch from 2cm to 1cm.
Removable support cannula, compatible with 10cm BD breast markers facilitates marker placement.
Three probe sizes available

Echogenic Markings Improve Visibility

TriConcave™ tip
Sharp needle for ease of penetration
Echogenic markings on cutting cannula
Enables visualization under ultrasound
Echogenic Markings
Improve Visibility

TriConcave™ tip
Sharp needle for ease of penetration
Echogenic markings on cutting cannula
Enables visualization under ultrasound
Top performance

LED light
Aids in visualizing biopsy site
Sample container
Illuminated sample container.
Enables you to visualize sample tissue for faster clinical decision-making.
Easy to remove and fits into a standard formalin container for transfer topathology lab.
Fluid management system
Prevents the movement of blood out of the probe and into the driver.
Absorb fluids as the tissue sample passes into the sample container.
Top performance

LED light
Aids in visualizing biopsy site
Sample container
Illuminated sample container.
Enables you to visualize sample tissuefor faster clinical decision-making.
Easy to remove and fits into a standard formalin container for transfer topathology lab.

Fluid management system
Manages fluid during the biopsy to simplify the cleanup procedure.

BD EleVation™ Driver charging
Completely charge the BD EleVation™ Driver prior to first use. The battery must be adequately charged prior to beginning each procedure.

- Ensure the appropriate AC adapter plug is installed on the AC power adapter.
- Connect the AC adapter cord to the wireless charging stand.
- Connect the AC adapter plug into a power source. If the wireless charging stand has current, the power light on the front of the wireless charging stand will illuminate green.
- Place the BD EleVation™ Driver in the wireless charging stand. The BATTERY indicator on the BD EleVation™ Driver will illuminate per the table to indicate the battery is charging.
A completely exhausted battery will require up to 12 hours to fully charge. After every use, and when the driver is not in use, the BD EleVation™ Driver should remain in the charging stand.
BD EleVation™ Breast Biopsy System
Indications for use 1. The BD EleVation™ Breast Biopsy System is indicated to obtain tissue samples from the breast or axillary lymph nodes for diagnostic analysis of breast abnormalities. The BD EleVation™ Breast Biopsy System is intended to provide breast tissue for histologic examination with partial or complete removal of the imaged abnormality. 2. The extent of histologic abnormality cannot be reliably determined from its mammographic appearance. Therefore, the extent of removal of the imaged evidence of an abnormality does not predict the extent of removal of a histologic abnormality, e.g. malignancy. When the sampled abnormality is not histologically benign, it is essential that the tissue margins be examined for completeness of removal using standard surgical procedures.
Contraindications 1. The BD EleVation™ Breast Biopsy System is for diagnostic use only, NOT for therapeutic use. 2. The BD EleVation™ Breast Biopsy System is contraindicated for those patients where, in the physician’s judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings 1. Patients who may have a bleeding disorder, or who are receiving anticoagulant therapy, may be at increased risk of complications. 2. As with any biopsy instrument, there is a potential risk for infection. 3. The BD EleVation™ Breast Biopsy System should not be used in a Magnetic Resonance Imaging (MRI) Suite. 4. The BD EleVation™ Breast Biopsy System has not been tested using stereotactic guidance or for use with an MRI. 5. The BD EleVation™ Breast Biopsy System should not be used in an operating room. 6. The BD EleVation™ Breast Biopsy System is not classified as an AP or APG device. 7. The BD EleVation™ Breast Biopsy System is not suitable for use in the presence of flammable anesthetic. 8. The BD EleVation™ Breast Biopsy System is not suitable for use in an oxygen rich environment. 9. The BD EleVation™ Driver must only be used with BD EleVation™ Probes and BD EleVation™ Accessories. 10. All breast biopsies should be performed under ultrasound guidance to confirm the BD EleVation™ Probe’s position relative to the target region to be sampled and to help mitigate the occurrence of a false negative biopsy. The BD EleVation™ Breast Biopsy System is intended for use with ultrasound imaging only. 11. The battery may only be replaced or disposed of by an authorized Service and Repair facility. 12. Use only with supplied AC power BD EleVation™ Accessories. Removing the AC adapter plug from wall power shall serve as isolation means. Do not position the AC adapter plug and wireless charging stand such that it is difficult to remove the AC adapter plug from the wall outlet if needed to remove mains power. 13. Do not reuse BD EleVation™ Probe. Reusing the BD EleVation™ Probe bears the risk of cross-patient contamination as biopsy probes, particularly those with long and small lumina, joints, and/or crevices between components, are dificult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the BD EleVation™ Probe for an indeterminable period of time. The residue of biological material can promote the contamination of the BD EleVation™ Probe with pyrogens or microorganisms which may lead to infectious complications. 14. Do not resterilize BD EleVation™ Probe. After resterilization, the sterility of the BD EleVation™ Probe is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilizing the BD EleVation™ Probe increases the probability that it will malfuntion due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
Precautions 1. The BD EleVation™ Breast Biopsy System should only be used by a physician trained in its indicated use, limitations, and possible complications of percutaneous needle techniques. 2. Do not attempt to remove the cover or modify the device in any way.
Potential Complications 1. Potential complications are those associated with any percutaneous removal/biopsy technique for tissue collection. Potential complications are limited to the region surrounding the biopsy site and include hematoma, lymphedema, hemorrhage, infection, non-healing wound, pain, nerve injury, and tissue adherence to the BD EleVation™ Probe while removing it from the breast. 2. As per routine biopsy procedures, it may be necessary to cut tissue adhering to the BD EleVation™ Probe while removing it from the breast.
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions and full directions for use.
![]()
