

Quick Reference Guide
Device description
The BD EleVation™ Breast Biopsy System is a handheld, selfcontained, single insertion, multiple sample biopsy device and is intended to be used with ultrasound guidance.
Loading and locking the BD EleVation™
Probe into the BD EleVation™ Driver
Step 1: insert probe
The BD EleVation™ Probe may be inserted into the driver in either of the following ways:
Option 1
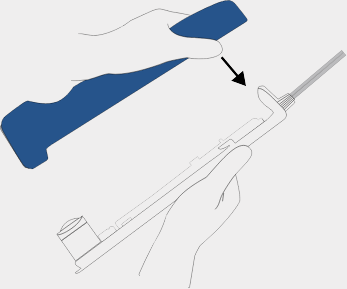
Insert probe
Remove the BD EleVation™ probe from the package using aseptic technique by grasping the BD EleVation™ probe from below.

Insert probe
Carefully align BD EleVation™ probe tabs and sample container with corresponding BD EleVation™ Driver slots.
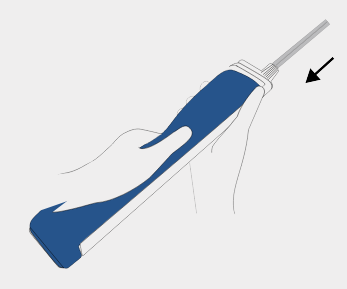
Insert probe
Slide the BD EleVation™ probe back to lock into place.
Option 2
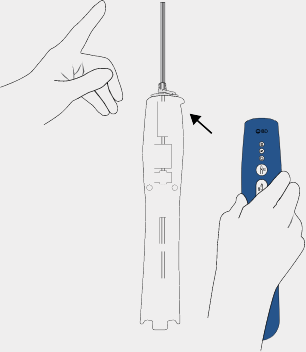
Insert probe
While the BD EleVation™ Probe remains in the tray packaging, align the BD EleVation™ Driver to the corresponding BD EleVation™ Probe tabs and sample container opening.
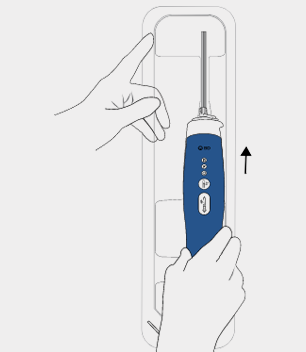
Insert probe
Slide the BD EleVation™ Driver forward to lock into place.
Step 2: Locking the probe
After loading the probe in one of the following ways shown above, confirm the BD EleVation™ Probe tab is locked into the BD EleVation™ Driver after loading.
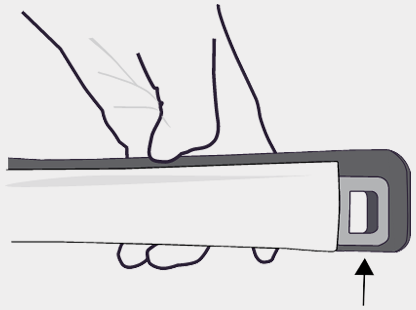
Removing the sample
Turn the sample container counterclockwise to remove from the driver.
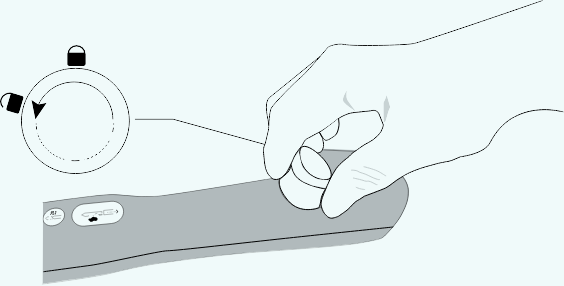
Rotate clockwise
Remove the tissue samples from the sample container by hinging it open. If additional samples are required, replace the sample container into the device by turning clockwise to lock into place. Ensure the sample container is secure before acquiring additional samples.

Sample container closed

Sample container hinges open
Removing the cannula
The support cannula may be detached and remain in the breast to retain a track to the biopsy site to place a breast tissue marker. To remove the support cannula from the BD EleVation™ probe, depress the coaxial arm and then twist the coaxial hub.
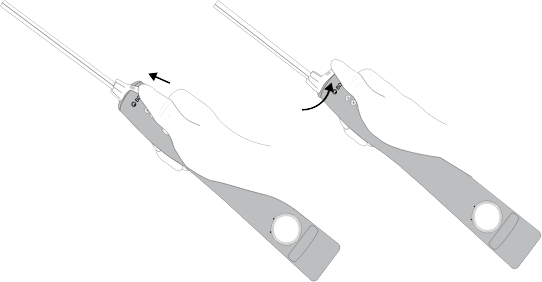
Removing the cannula
The support cannula may be detached and remain in the breast to retain a track to the biopsy site to place a breast tissue marker.
Removing the cannula
To remove the support cannula from the BD EleVation™ probe, depress the coaxial arm and then twist the coaxial hub.
Removing the probe
Remove the BD EleVation™ Probe from the BD EleVation™ Driver by pressing down on the locking tab, sliding the BD EleVation™ Probe cover completely forward and then pulling the BD EleVation™ Probe straight up from the BD EleVation™ Driver.
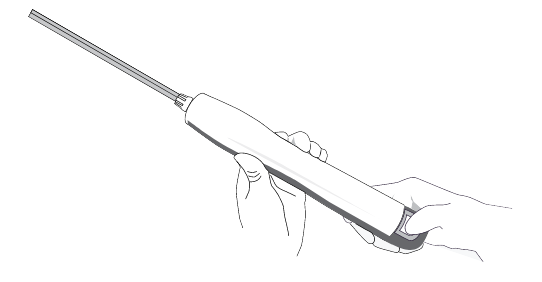
Removing the probe
Press the locking tab
Cleaning and maintenance
After each use, wipe the BD EleVation™ Driver with a Super Sani-Cloth, Super Sani-Cloth Plus or a cloth dampened with water to remove any excess blood or fluids present on the device.
Following the initial wipe, throughly clean the BD EleVation™ Driver with Super Sani-Cloths, or Super Sani-Cloth Plus paying particular attention to the buttons and indicators, locking tab, LED lights, internals gears, and the sides of the driver.
Allow the driver to remain wet for a minimum of 3 minutes. Allow the BD EleVation™ Driver to air dry throughly before placing in the wireless charging stand.
Other than routine cleaning, no other preventative maintenance is required. However, it is recommended to return the device to an authorized Service and Repair Facility once per year for inspection and servicing.
Sterilization and exposure to fluids may damage the electrical components of the device. If the BD EleVation™ Driver is inappropriately cleaned, it may cause the BD EleVation™ Driver to malfunction and will void the standard warranty.
Do not autoclave. Do not heat over 54º (129º F).
The wireless charging stand may be cleaned with the same methods as the BD EleVation™ Driver.
BD EleVation™ Driver charging
Battery status
Solid Green
Battery status
Battery has sufficient charge to complete ONE biopsy procedure.
Action required
No action needed. BD EleVation™ Breast Biopsy System is ready to perform a biopsy.
Blinking green
Battery status
Battery has sufficient charge to complete ONE biopsy procedure.
Action required
Proceed with performing the biopsy. After completing the procedure the BD EleVation™ Driver MUST be charged prior to the next biopsy.
Solid Red
Battery status
Battery is NOT sufficently charged to perform a biopsy procedure.
Action required
Return BD Elevation™ Driver to charging stand to charge battery.
Battery LED is not illuminated – Driver does not respond when probe is loaded
Battery status
Battery is completely discharged.
Action required
Return BD Elevation™ Driver to charging stand to charge battery.
After every use, and when the driver is not in use, the BD EleVation™ Driver should remain in the charging stand. A regulator restricts overcharging the battery.
Troubleshooting
Condition
Low battery
Action required
Place BD EleVation™ Driver on wireless charging stand to charge battery.
BATTERY indicator is solid red

Condition
Error during:
• Calibration
• PRIME/PIERCE
• Sampling
Action required
• Remove BD EleVation™ Probe
• Place BD EleVation™ Driver on a flat surface with hand away from any moving components
• Press SAMPLE button
ERROR indicator blinks red, PRIME/PIERCE and SAMPLE buttons are solid red

Condition
Smart Mode overloaded
Action required
• Remove BD EleVation™ Probe
• Place BD EleVation™ Driver on a flat surface with hand away from any moving components
• Press SAMPLE button
ERROR indicator blinks red, SMART MODE indicator blinks red, PRIME/PIERCE and SAMPLE buttons are solid red

Condition
BD EleVation™ Driver placed in wireless charging stand while BD EleVation™ Probe is loaded
Action required
• Remove BD EleVation™ Driver from wireless charging stand
• Remove BD EleVation™ Probe
BATTERY indicator blinks red, ERROR indicator blinks red, PRIME/PIERCE and SAMPLE buttons are solid red

Condition
BD EleVation™ Driver placed in wireless charging stand while in error
Action required
• Remove BD EleVation™ Driver from wireless charging stand
• Remove BD EleVation™ Probe
• Place BD EleVation™ Driver on a flat surface with hand away from any moving components
• Press SAMPLE button
BATTERY indicator blinks red, ERROR indicator blinks red, PRIME/PIERCE and SAMPLE buttons are solid red
NOTE: Driver is also beeping continuously
BD EleVation™ Breast Biopsy System
Indications for use 1. The BD EleVation™ Breast Biopsy System is indicated to obtain tissue samples from the breast or axillary lymph nodes for diagnostic analysis of breast abnormalities. The BD EleVation™ Breast Biopsy System is intended to provide breast tissue for histologic examination with partial or complete removal of the imaged abnormality. 2. The extent of histologic abnormality cannot be reliably determined from its mammographic appearance. Therefore, the extent of removal of the imaged evidence of an abnormality does not predict the extent of removal of a histologic abnormality, e.g. malignancy. When the sampled abnormality is not histologically benign, it is essential that the tissue margins be examined for completeness of removal using standard surgical procedures.
Contraindications 1. The BD EleVation™ Breast Biopsy System is for diagnostic use only, NOT for therapeutic use. 2. The BD EleVation™ Breast Biopsy System is contraindicated for those patients where, in the physician’s judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings 1. Patients who may have a bleeding disorder, or who are receiving anticoagulant therapy, may be at increased risk of complications. 2. As with any biopsy instrument, there is a potential risk for infection. 3. The BD EleVation™ Breast Biopsy System should not be used in a Magnetic Resonance Imaging (MRI) Suite. 4. The BD EleVation™ Breast Biopsy System has not been tested using stereotactic guidance or for use with an MRI. 5. The BD EleVation™ Breast Biopsy System should not be used in an operating room. 6. The BD EleVation™ Breast Biopsy System is not classified as an AP or APG device. 7. The BD EleVation™ Breast Biopsy System is not suitable for use in the presence of flammable anesthetic. 8. The BD EleVation™ Breast Biopsy System is not suitable for use in an oxygen rich environment. 9. The BD EleVation™ Driver must only be used with BD EleVation™ Probes and BD EleVation™ Accessories. 10. All breast biopsies should be performed under ultrasound guidance to confirm the BD EleVation™ Probe’s position relative to the target region to be sampled and to help mitigate the occurrence of a false negative biopsy. The BD EleVation™ Breast Biopsy System is intended for use with ultrasound imaging only. 11. The battery may only be replaced or disposed of by an authorized Service and Repair facility. 12. Use only with supplied AC power BD EleVation™ Accessories. Removing the AC adapter plug from wall power shall serve as isolation means. Do not position the AC adapter plug and wireless charging stand such that it is difficult to remove the AC adapter plug from the wall outlet if needed to remove mains power. 13. Do not reuse BD EleVation™ Probe. Reusing the BD EleVation™ Probe bears the risk of cross-patient contamination as biopsy probes, particularly those with long and small lumina, joints, and/or crevices between components, are dificult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the BD EleVation™ Probe for an indeterminable period of time. The residue of biological material can promote the contamination of the BD EleVation™ Probe with pyrogens or microorganisms which may lead to infectious complications. 14. Do not resterilize BD EleVation™ Probe. After resterilization, the sterility of the BD EleVation™ Probe is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilizing the BD EleVation™ Probe increases the probability that it will malfuntion due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
Precautions 1. The BD EleVation™ Breast Biopsy System should only be used by a physician trained in its indicated use, limitations, and possible complications of percutaneous needle techniques. 2. Do not attempt to remove the cover or modify the device in any way.
Potential Complications 1. Potential complications are those associated with any percutaneous removal/biopsy technique for tissue collection. Potential complications are limited to the region surrounding the biopsy site and include hematoma, lymphedema, hemorrhage, infection, non-healing wound, pain, nerve injury, and tissue adherence to the BD EleVation™ Probe while removing it from the breast. 2. As per routine biopsy procedures, it may be necessary to cut tissue adhering to the BD EleVation™ Probe while removing it from the breast.
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions and full directions for use.
![]()
